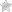When comparing soda ash vs. baking soda, it's important to know what they are, how they're used, and how they're made. Soda ash is a form of sodium carbonate, while baking soda is a refined form of soda ash called sodium bicarbonate. Baking soda is commonly found in pantries, while one of the most common applications for soda ash has to do with raising the pH levels in chlorine pools. Along with many other practical applications, these two household items have different chemical makeups, unique properties, and varying applications. While there is some crossover, they are decidedly different things.
In this post, we'll talk about soda ash and baking soda in terms of similarities and differences. You'll learn exactly what differentiates soda ash from baking soda and get some insight into their chemical makeup and creation. We'll also talk about their histories, practical applications, and popular brands to buy. Keep on reading to go on a deep dive into the major differences between soda ash and baking soda.
Soda Ash vs. Baking Soda: What's the Difference?
Perhaps the biggest immediate difference between soda ash and baking soda is in their name. Soda ash is known as sodium carbonate, which refers to its chemical makeup, while baking soda refers to sodium bicarbonate. Soda ash is a highly alkaline pH regulator that is used in everything from photography to washing and glass production to the creation of Sherbert powder for sweet and tasty treats. Baking soda, on the other hand, is known as a chemical leavening agent, which means it causes things to rise and bubble with the production of carbon dioxide. It is used in cleaning, baking, and homeopathic fixes. Let's get into some of the finer details of soda ash vs. baking soda.
What is Soda Ash?
Soda ash, or Na2CO3, is a refined chemical version of a naturally occurring process. It is produced in three major areas of industry. In relation to food, soda ash is developed as an additive and is also an ingredient in the animal feed industry. In terms of industrial and “technical grade” soda ash, it is used in water treatment, cleaning products, glass, leather, and paper production (via Kazan Soda).
History and Origin of Soda Ash
Soda ash has been used for thousands of years. Soda ash forms in a couple of natural ways, either over long periods of time or with some helpful nudging. One way it forms is in desert lakes, where the process of burning plants can react with the sodium and carbon dioxide in the soil to create ash. The ancient Egyptians used this ash and its basic properties to lower the melting point for materials used to create glass structures. Meanwhile, the Romans found uses for this ash in baking, cleaning, and medicine. Synthetic production for soda ash didn't develop until the 1800s. In 1998, soda ash was number 11 on a list of the top inorganic chemicals in production worldwide.
How Soda Ash is Made
Naturally, soda ash deposits, which are called Trona, are formed when igneous rocks break down and react to the carbon dioxide of the soil, leaving soda ash in their wake (via Leaf TV). They can also be formed in a reaction of burning plants on desert soil, as mentioned above. According to Kazan Soda, there are major Trona deposits in Wyoming, USA, parts of Turkey, and some minimal deposits in China. Synthetic soda ash is produced in one of two production methods. These methods are called the Solvay or Hou methods. Though they differ slightly, the basic reaction is between salt (sodium chloride) and limestone or ammonia, which results in soda ash. Around 67% of soda ash produced around the world is synthetically made.
Popular Uses For Soda Ash
We've mentioned a couple of major uses above, but to best demonstrate the variety of uses for soda ash, please enjoy this bulleted list:
- It is used in film development to balance out pH levels
- It is also used in pools to balance out pH levels
- Cleaning solutions often contain sodium carbonate
- It's used in water treatment to soften harsh calcium and magnesium compounds
- It's used in the paper, glass, and leather industry
- Sodium carbonate is used as an additive for many types of foods and additives
- Sodium carbonate is used for the manufacturing of glass, paper, and soup
- As mentioned above, it can lower the melting point of ingredients like silica
What is Baking Soda?
Baking soda is also known as sodium bicarbonate and is commonly used in baking, cleaning, and for medicinal purposes. For a classic show of baking soda's carbon makeup, combine it with vinegar for a volcanic eruption. Let's get more into the chemical makeup, applications, and history of baking soda.
History and Origin of Baking Soda
Baking soda was first processed in the 1790s by a scientist named Nicolas Leblanc. By the 1840s, two brothers named Austin Church and John Dwight developed the brand “Arm & Hammer Church & Co’s bicarbonate of soda.” Still a major leader in baking soda distribution today, Arm & Hammer distributed cookbooks to illustrate the many ways baking soda could be used beyond just cleaning and medicine. By the 1920s, baking soda was a staple in fridges and pantries for freshening, leavening, and cleaning (via Everyday Health).
How Baking Soda is Made
Baking soda's chemical formula is NaHCO3. Like soda ash, it is extracted from trona deposits and then further processed into baking soda. This refining process entails combining carbon dioxide and ammonia through a salt solution. The resulting alkaline powder has amazing chemical leavening properties.
Popular Uses For Baking Soda
Baking soda can be used for cleaning, baking, and healing. It helps to treat bee stings, mosquito bites, and heartburn. It can neutralize odors and acids and helps in cleaning corroded and rusty metals. Like soda ash, baking soda has numerous applications (via Healthline). However, it's used predominantly in baking. Here's a list of some of the many uses for baking soda:
- It can be used as a mouthwash or teeth whitener
- Baking soda is an ingredient in natural deodorant and can be used to keep your fridge fresh
- Like whitening your teeth, baking soda can be used to whiten laundry
- As a cleaning agent, it has many practical uses; try it out anywhere in your house!
- It can be used to treat stains
- Baking soda makes an easy wash for fruits and veggies
- Use it to polish silverware or scorched pots
- It can be diluted with water and sprayed on weeds to kill them naturally
- It can relieve sunburns, heartburns, stomach pains, and itchy bug bites
Soda Ash vs. Baking Soda: Are They The Same Thing?
The short version is that baking soda is a refined version of soda ash. However, they have different applications and chemical makeups despite being mined from the same natural resource. Their synthetic processes are also the same. However, the results are two alkaline powders with different applications and unique properties. While soda ash is present in a number of industries, baking soda is mostly used in baking, cleaning, and homeopathic settings.
Possible Alternatives To Soda Ash And/Or Baking Soda
Some alternatives to soda ash or baking soda for washing include white vinegar and liquid fabric softeners. In terms of baking, self-rising flour, potassium bicarbonate, whipped egg whites, aquafaba, club soda, baker's ammonia, and baking powder are all reasonable substitutes. But do your research because some recipes will not work with these exchanges and may change the cooking time, texture, and overall result of what you're intending to make. Best of luck!
Comparison of Soda Ash vs. Baking Soda
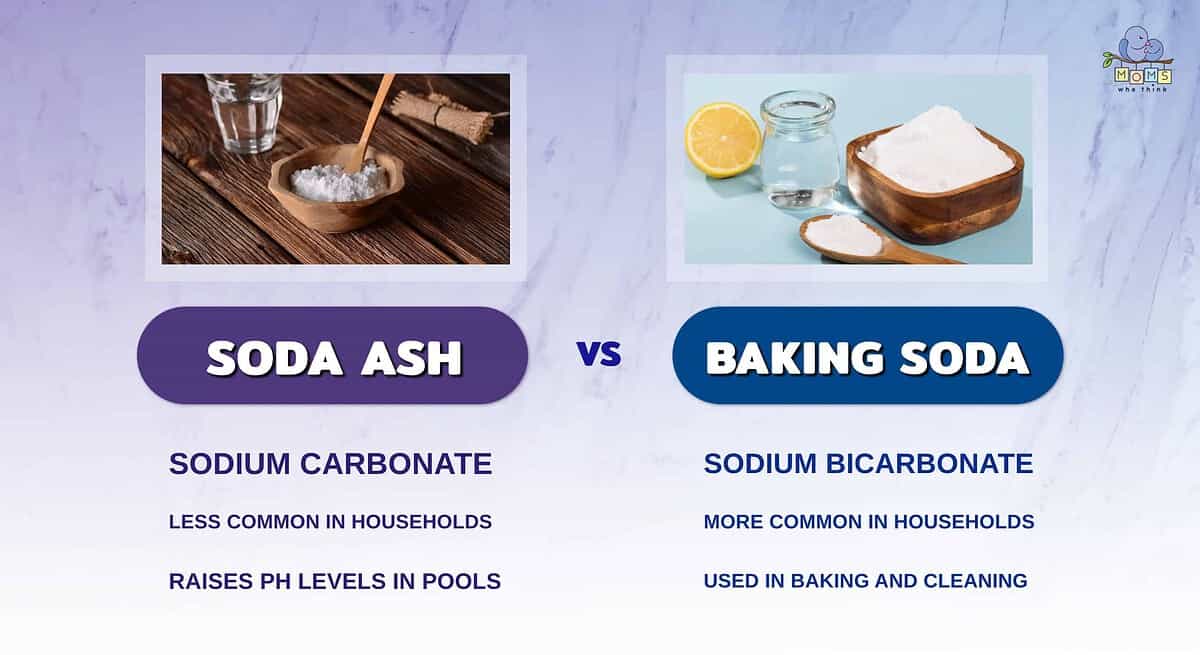
Soda ash and baking soda may look very similar, but they actually serve completely different purposes. Let's do a recap of this article to reveal a few of these distinctions:
- Soda ash is basically sodium carbonate, while baking soda is sodium bicarbonate, making their chemical properties completely different.
- Baking soda is commonly used for cleaning, baking, and medicinal purposes. On the other hand, soda ash is known to balance pH levels in pools and film development, as well as other commercial products.
- While both are great household products, soda ash is less commonly used in households than baking soda, which is considered a nearly everyday product.
Baking with Baking Soda
Recipes like muffins, breads, cookies, and cakes include baking soda in their ingredients. There is a chemical reaction that happens to baking soda when it is being baked that helps baked goods to rise and gives them a fluffy and light texture. This giant cookie pizza recipe uses baking soda to make it light and fluffy. You can add whatever toppings you'd like and enjoy it as pizza slices or as one giant cookie.
Print
Giant Cookie Pizza
Ingredients
- 1/2 cup white sugar
- 1/2 cup packed light brown sugar
- 1/2 cup butter, melted
- 1 egg
- 1/2 teaspoon baking soda
- 1/2 teaspoon salt
- 1/2 teaspoon vanilla
- 1 1/2 cup flour
- Heaping 1/2 cup chocolate chips, M&M's, or whatever candy you desire
Instructions
- Preheat the oven to 350 degrees.
- Cream together the butter and sugars, mix in eggs, and vanilla.
- Next add in the flour, salt, and baking soda. Then fold in the chocolate chips and candies.
- Refrigerate the dough for at least 1-2 hours. This allows the melted butter to firm up a bit. If you skip this step it may cause your cookie dough to spread a bit too much during baking.
- Use a silicone baking mat (like this one here) and press the cookie dough into a 9 inch circle and about 1/2 inch thick. The cookie will expand/spread a bit while cooking so don't spread out too thin!
- Bake for 13-16 minutes or until the cookie is a light golden brown. Do not remove the cookie from the baking mat until it is completely cooled. It will continue to bake and firm up as it cools and will lift off of the pan more easily without breaking if you wait until it has completely cooled.
- Add additional toppings if desired and cut into pizza slices! You can also leave it as a giant cookie and make someone's day by taking it to them as a gift! Enjoy!
The image featured at the top of this post is ©iStock.com/JPC-PROD.